Tricks To Remember Amino Acids: In our daily life, we come across many chemical compounds, which are necessary for the growth and development of our bodies. Amino acids are also one among them. Hence here we are going to cover Amino acids in detail with tricks to remember them as well.
I know you are looking for tricks to remember amino acids. Don’t worry we are going to give you some of the best and unique tricks to remember the Amino Acids. You can click and download the PDF and print for your convenience and revisions.
So before going to get the tricks to remember amino acids, you must know some basic terminologies associated with Amino acids only then you will be able to correlate with the Amino Acids. Hence let’s understand the terms first…
What are Amino Acids?
According to Britanica.com
Amino acid, any of a group of organic molecules that consist of a basic amino group (―NH2), an acidic carboxyl group (―COOH), and an organic R group (or side chain) that is unique to each amino acid. The term amino acid is short for α-amino [alpha-amino] carboxylic acid. Each molecule contains a central carbon (C) atom, called the α-carbon, to which both an amino and a carboxyl group are attached. The remaining two bonds of the α-carbon atom are generally satisfied by a hydrogen (H) atom and the R group. The formula of a general amino acid is:
Other Definitions:
Amino Acids are organic compounds that combine and form proteins. Hence also called building blocks of proteins. They are involved in many biological and chemical functionalities inside our bodies. They are very essential for the growth and development of the human body.
More than 300+ amino acids are there in nature.
Also Read: Tricks To Remember ASEAN Countries
Below are some important amino acids with their chemical formulas are mentioned:
| Alanine | C3H7NO2 | Leucine | C6H13NO2 |
| Aspartic Acid | C4H7NO4 | Lysine | C6H14N2O2 |
| Asparagine | C4H8N2O3 | Methionine | C5H11NO2S |
| Arginine | C6H14N4O2 | Proline | C5H9NO2 |
| Cytosine | C4H5N3O | Phenylalanine | C9H11NO2 |
| Cysteine | C3H7NO2S | Serine | C3H7NO3 |
| Glycine | C2H5NO2 | Tyrosine | C9H11NO3 |
| Glutamine | C5H10N2O3 | Threonine | C4H9NO3 |
| Histidine | C6H9N3O2 | Tryptophan | C11H12N2O2 |
| Isoleucine | C6H13NO2 | Valine | C5H11NO2 |
Tricks To Remember Amino Acids:
The mnemonic for memorizing the names of the essential amino acids:
Look There, Look There, VIP Man
The essential amino acids are:
• Leucine
• Threonine
• Lysine
• Tryptophan
• Valine
• Isoleucine
• Phenylalanine
• Methionine
You Can Also Try To Remember In Another Way As Well:
• I: Isoleucine
• Love: Leucine
• Lucy: Lysine
• Very: Valine
• Much: Methionine
- Please: Phenylalanine
- Try: Tryptophan
- To: Threonine
- Help: Histidine (Semi-essential)
- Arginine: Arginine (Semi-essential)
So, knowing tricks is not enough you must know some basic properties of amino acids as well. You can read further in the below section:
Basic Properties of Amino Acids:
Physical Properties
- Amino acids are colorless, crystalline solid.
- All amino acids have a high melting point greater than 200o
- Solubility: They are soluble in water, slightly soluble in alcohol, and dissolve with difficulty in methanol, ethanol, and propanol. R-group of amino acids and pH of the solvent play important role in insolubility.
- On heating to high temperatures, they decompose.
- All amino acids (except glycine) are optically active.
- Peptide bond formation: Amino acids can connect with a peptide bond involving their amino and carboxylate groups. A covalent bond formed between the alpha-amino group of one amino acid and an alpha-carboxyl group of other forming -CO-NH-linkage. Peptide bonds are planar and partially ionic.
Chemical Properties
- Zwitterionic property
A zwitterion is a molecule with functional groups, of which at least one has a positive and one has a negative electrical charge. The net charge of the entire molecule is zero. Amino acids are the best-known examples of zwitterions. They contain an amine group (basic) and a carboxylic group (acidic). The -NH2 group is the stronger base, and so it picks up H+ from the -COOH group to leave a zwitterion. The (neutral) zwitterion is the usual form of amino acids that exist in the solution. - Amphoteric property
Amino acids are amphoteric in nature that is they act as both acids and base due to the two amine and carboxylic groups present. - Ninhydrin test
When 1 ml of Ninhydrin solu¬tion is added to a 1 ml protein solution and heated, the formation of a violet color indicates the presence of α-amino acids. - Xanthoproteic test
The xanthoproteic test is performed for the detection of aromatic amino acids (tyrosine, tryptophan, and phenylalanine) in a protein solution. The nitration of benzoin radicals present in the amino acid chain occurs due to a reaction with nitric acid, giving the solution yellow coloration. - Reaction with Sanger’s reagent
Sanger’s reagent (1-fluoro-2, 4-dinitrobenzene) reacts with a free amino group in the peptide chain in a mild alkaline medium under cold conditions. - Reaction with nitrous acid
Nitrous acid reacts with the amino group to liberate nitrogen and form the corresponding hydroxyl.
What are Essential and Nonessential Amino Acids?
The human body can synthesize some amino acids hence they are called non-essential amino acids.
They are:
alanine, asparagine, arginine, aspartic acid, glutamic acid, cysteine, glutamine, proline, glycine, serine, and tyrosine.
There are some which are not synthesized by our body hence are called essential amino acids.
They are:
Isoleucine, histidine, lysine, leucine, phenylalanine, tryptophan, methionine, threonine, and valine
Structure of amino acids:
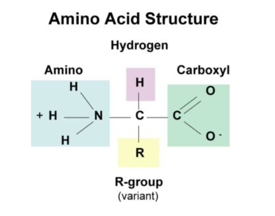
The general structure of Amino acids is H2NCH RCOOH and it can be written as:
COOH
|
H2N – – C – – H
|
R
There are 20 naturally occurring amino acids and all have common structural features – an amino group (-NH3+), a carboxylate (-COO-) group, and a hydrogen-bonded to the same carbon atom. They differ from each other in their side-chain called the R group. Each amino acid has 4 different groups attached to α- carbon.
These 4 groups are:
• Amino group,
• COOH,
• Hydrogen atom,
• Sidechain (R).
Here is the structure of twenty amino acids with their chemical formula.

The human body can synthesize only a certain amount of amino acids and the rest which are called essential amino acids should be supplied through protein-rich foods.
They are:
plant-based products like broccoli, beans, beetroots, pumpkin, cabbage, nuts, dry fruits, chia seeds, oats, peas, carrots, cucumber, green leafy vegetables, onions, soybeans, whole grain, peanuts legumes, lentils, etc. Fruits rich in amino acids are apples, bananas, berries, figs, grapes, melons, oranges, papaya, pineapple, and pomegranates. Other animal products include dairy products, eggs, seafood, chicken, meat, pork, etc.
What are essential Amino Acids:
Those amino acids are present in food and cannot be synthesized by our body.
A Brief About Tricks To Remember Amino Acids PDF
| Category | Educational |
| Number of Pages | 07 |
| Year | 2021 |
| Size of PDF | 1.3 MB |
| Language | English |
| Credit Source | epaperpdf.com |
Here is Tricks To Remember Amino Acids PDF
More PDFs Like This:
| Important Acts For UPSC 2021 PDF Download |
| Kanishak Kataria’s Vajiram Class Notes PDF Download |
| Constitution Of India PDF Download |
Important Note:
We are not the owner of the book nor we publish it in any way. We are sharing the links which are available on the internet. If you found it to be violating any of your copyright rights then email us at epaperpdffree@gmail.com.
We will revert you ASAP and take down the book. Thanks for cooperating.





